Background: Liposomal Daunorubicin-Cytarabine (CPX-351 or Vyxeos), is approved for upfront treatment of adult and pediatric patients with secondary Acute Myeloid Leukemia (sAML) based on results of a randomized phase 3 trial comparing CPX-351 to conventional chemotherapy with 7+3 regimen. The study showed CPX-351 treated patients demonstrated superior complete remission (CR) rates and overall survival (OS) [Lancet et al JCO 2018]. In this study, a higher proportion of CPX-351 treated patients underwent allogeneic hematopoietic cell transplantation (alloHCT), and landmark OS analysis favored CPX-351 treated patients (HR=0.46; 95% CI: 0.24-0.89; p=0.009). Herein, we reviewed City of Hope (COH) and University of California Los Angeles Medical Center (UCLA) database for patients with sAML who underwent allogeneic HCT after CPX-351 induction to investigate OS and disease-free survival (DFS) outcomes of alloHCT in CPX-351 treated patients and to investigate if HCT outcomes are impacted by achievement of pre-HCT measurable residual disease (MRD) negative remission.
Methods: We conducted a multi-center retrospective review of 38 consecutive patients who received CPX-351-based induction before allogeneic HCT to treat sAML, at COH (n=10) and UCLA (n=28), between 2017-2021. The primary endpoint was 2-year OS and DFS in all patients. Secondary outcomes included MRD-ve complete remission (CR) after CPX-351 induction. CR was defined as <5% blasts in bone marrow (BM) aspirates. CR with with hematologic recovery (CRh) was defined as absolute neutrophil counts >1000/µL and platelets ≥100,000/µL. Patients without CRh were categorized as CR with incomplete blood recovery (CRi). MRD assessment was done on day-28 BM aspirate using multiparametric flow cytometric (FC) assay with lower limit of sensitivity of 0.01%. OS was defined as the time from the start of therapy to death and patients were censored if alive at the last follow-up, DFS was defined as the time interval from the date of response to relapse or death, whichever occurred first, and patients were censored at the last follow-up if still leukemia free. Descriptive statistics were used to summarize patient demographics, and disease characteristics. Kaplan-Meier curves and log-rank test were used to evaluate OS and LFS.
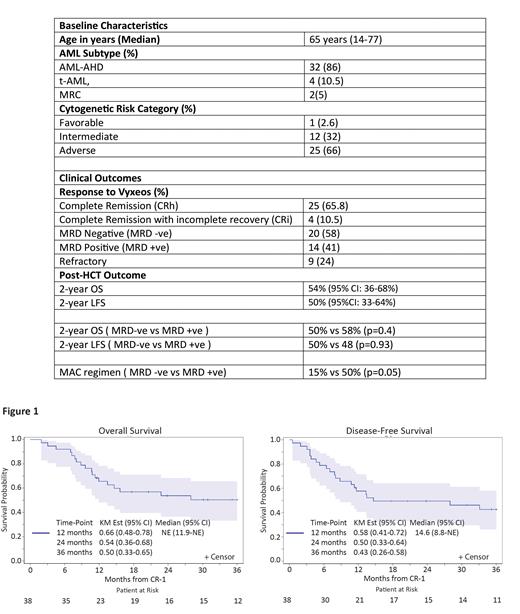
Results: Patients' demographic and disease features are summarized in Table 1. Briefly, the median age at diagnosis was 65 years (range: 14-77) and median blast percentage in BM at diagnosis was 34% (range: 20-69%). Based on ELN criteria, 25 patients (66%) had adverse risk, 12 (32%) had intermediate risk and one (2.6%) had favorable risk AML. sAML was due to antecedent hematologic disorder (AHD) in majority of patients (n=32; 84%), therapy related in four (10%) and MDS-related changes (MRC) in two patients (5.3%). Following Vyxeos treatment, the composite CR rate (CRh+CRi) was 76% (CRh in 25, CRi in 4 patients) and 9 patients (23%) were refractory to upfront therapy. Pre-HCT MRD status was available for majority of patients (n=34; 89%). MRD negative remission by FC was achieved in 58% (20/34) patients (Table 2).
The 1- and 2-years OS was 65% (95% CI: 48-78%) and 54% (95% CI: 36-68%), respectively and the 1- and 2-years DFS was 58% (95% CI: 41-71) and 50% (95%CI: 33-64%), respectively (Figure 1). In the 34 patients with available pre-HCT MRD data, no significant difference in OS (50% vs 58%; p=0.4) or DFS (50% vs 48%; p=0.93) was observed based on pre-HCT MRD status. More patients with who were MRD-positive after induction (50%; 7/14) received myeloablative conditioning (MAC) regimen compared to only 15% (3/20) of patients with MRD negative remission.
Conclusion: CPX-351 induction in patients with sAML is associated with high MRD negative remission and promising 2-year OS/DFS after allogeneic HCT. In patients with MRD positive remission status after CPX-351 induction, MAC conditioning regimens may be preferred to reduce relapse incidence after alloHCT.
Disclosures
Marcucci:Ostentus Therapeutics: Current equity holder in private company, Research Funding. Nakamura:International Consortium: Other: consortium chair; Omeros: Consultancy; Napajen: Consultancy; Blue Bird: Consultancy; Miyarisan: Research Funding; Jazz Pharmaceuticals: Consultancy, Other: research collaboration; Mt. Sinai: Other: Acute GVHD; Sanofi: Consultancy; NCCN: Other: guideline panel for HCT; Leukemia & Lymphoma Society: Other: grant reviewer; BMT CTN Steering Committee: Membership on an entity's Board of Directors or advisory committees; NCTN Lymphoma Steering Committee: Membership on an entity's Board of Directors or advisory committees. Pullarkat:Servier: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Genentech: Consultancy, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau. Oliai:Seagen: Research Funding; Arog: Research Funding; Jazz Pharmaceuticals: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Orca Bio: Research Funding. Salhotra:OrcaBio: Research Funding; Sanofi: Speakers Bureau; Rigel Pharma: Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Research Funding; Kura Oncology: Research Funding; Gilead: Research Funding; BMS: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal